|
AIDS Treatment News
Grossman: It has been poorly communicated to patients that our great desire would be to put everybody on therapy when we first find out they were infected, if we had therapies that were not toxic, easy to take, and long-lasting. But given the fact that we don't, we settled on the 350 T-cell count as a general guideline for starting antiretrovirals. It keeps bearing up in study after study that it is a pretty good place to set that line. But when you start looking at much less toxic drugs like tenofovir, or at somebody who could take a triple nucleoside regimen that does not have really bad toxicity, and might be able to take that treatment for years, it reopens the question of when to start. Nothing is set in stone in HIV medicine. No question is forever closed. ATN: What are doctors combining tenofovir with? Grossman: Tenofovir with abacavir [ZIAGEN(R)] and 3TC [EPIVIR(R)] is a good regimen, all once-a-day. Or tenofovir with ddI [VIDEX(R)] and 3TC, although some people worry that you might be more likely to develop a K65R mutation, which could affect other nucleoside analogs. Or use a non-nucleoside that does not have major side effects. Less toxic regimens re-open the question about when is the best time to start antiretrovirals. ATN: For the non-nucleoside, would you choose efavirenz [SUSTIVA(R)], for someone who does not have a big problem with the mood changes or other mental effects? Grossman: I like efavirenz, but we are using more nevirapine [VIRAMUNE(R)]. I think with once-a-day therapy, morning dosing works better than nighttime dosing. Most patients are more likely to forget to take a dose at night. ATN: You haven't had viral breakthroughs with nevirapine? Grossman: No, we have seen good efficacy. And a number of studies at the Barcelona international conference showed pretty comparable efficacy between nevirapine and efavirenz. The problem is that Boehringer-Ingelheim has not done the studies. Those at the international conference were done by private investigators, and not supported by Boehringer Ingelheim. The company dropped the ball on getting answers to the questions. ATN: I had the impression that people were choosing efavirenz as their first choice. Grossman: I don't think that is necessary. Efavirenz is certainly a fine choice for people who can tolerate it, and I do have lots of patients on it. But again, with once-a-day, I think that morning dosing works better. ATN: What do you typically combine with nevirapine in a starting regimen? Grossman: Often I put people on nevirapine, 3TC, and tenofovir. With this regimen few patients will have major side effects. There can be problems, but not as much as with other drugs. ATN: And with nevirapine, it's the rash, and you have to watch the liver enzymes? Grossman: Right. And nobody can tell who is going to have the acute hepatic syndrome. Today that is totally unpredictable. But it is rare. There have only been a handful of cases reported of acute hepatic necrosis. This seems to be an unpredictable, rare, and very acute syndrome that has happened in some people with very high CD4 counts. The liver toxicity that is more common is an asymptomatic rise in liver enzymes that occurs in the first 6 weeks of treatment. If people develop elevated liver enzymes and symptoms, they can stop the drug and the symptoms disappear. And if they make it past the six weeks they probably won't get this problem. That is why Boehringer-Ingelheim has recommended that people starting Nevirapine get their liver function tests done every 2 weeks for the first 6 weeks. ATN: Any other thoughts about regimens for starting antiretroviral therapy? Grossman: I strongly believe in once-a-day therapy. I consider it the big breakthrough of the last year and next year. It is being accepted in a reverse of the usual way. Usually in HIV, the first people to develop new strategies are investigators and academics. In this case, however, the investigators have been holding back on once-a-day therapy. But it has caught on in a huge way in the community. For the doctors in the trenches who have patients who are difficult to treat, it has been a no-brainer that once-a-day is the way to go. In Barcelona there were two posters about patients preferring once-a-day therapy. Both were studies sponsored by Bristol-Myers Squibb, which has a big stake in promoting once-a-day. But many of the research physicians have been holding back. For my patients it has made a tremendous difference. We offer once-a-day to everybody who has a possibility of taking it -- even if that means changing their regimen, because I think it makes such a difference in terms of increased adherence, increased efficacy and patient satisfaction. At Barcelona I presented a poster on once-a-day, looking at the first 50 patients we treated, and it was successful. But there was not much information at the conference about qd [once-a-day] therapy. There was interesting information about ritonavir/saquinavir. One poster compared it vs. indinavir/ritonavir, both twice a day in this trial [P. Cahn and others, abstract #WeOrB1265], and the number of adverse events, change in LDL cholesterol, and change in triglycerides, was lower in the ritonavir/saquinavir group. Yet they did just as well virologically. Another poster looked at switching patients from another protease inhibitor regimen that failed to a ritonavir/saquinavir protease inhibitor regimen [J.G. Baril and others, #WePeB5902]. This retrospective chart review of 12 patients found generally good results. But so far I have not had good success with once-a-day protease inhibitors. I had a couple patients on amprenavir/ritonavir once-a-day, and a couple patients on saquinavir/ritonavir once-a-day, but that takes lots of pills. When we get newer protease inhibitors, like atazanavir and GSK908, once-a-day PIs will become practical. There was also a followup to the Kaletra (lopinavir/ritonavir) once-a-day vs. twice a day trial in treatment-naive patients [J. Feinberg and others, #TuPeB4445]. Now they are reporting at 72 weeks, and there was no significant difference between the arms in virologic suppression. But in this study, trough levels on the twice-daily Kaletra regimen cluster very close to 100 times the IC-50 [meaning that the lowest drug level in the blood, usually just before the patient takes the next dose, is about 100 times the amount needed to inhibit 50% of the virus] -- a good place. Trough levels on once-a-day have much more variation. Some patients are much closer to the IC-50. I tried this Kaletra regimen in 5 patients who were not treatment-naive, and we measured their lopinavir trough levels after 2 weeks, and all 5 were below the IC-50. So I took everybody off once-a-day Kaletra. While the trial seems to be achieving good results, I am not sure that it is expandable beyond naive patients. I don't think people should try this outside of a study. ATN: What do you think about continuing antiretroviral therapy in heavily treated patients who cannot control the virus with any currently available drugs? Grossman: I have advanced patients who have a viral load over 750,000 copies -- they are definitely not controlling the virus. I think they still get some benefit from the drugs. But their T-cells are too low to show if there is a response. Usually if they do not have overwhelming side effects, I recommend that they stay on the drugs. I think from Steve Deeks' data a couple years ago and some other studies, that patients get some benefit from antiretrovirals even if the virus is resistant and cannot be well controlled with the drugs. Lipodystrophy ATN: On lipodystrophy, what are you seeing? What drugs or combinations seem to be associated more with the problem?
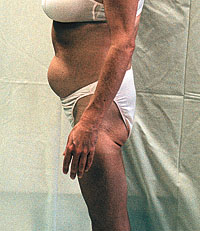 Grossman: I see both fat accumulation and fat wasting. Fat accumulation is
sometimes hard to diagnose. I use what I call the pinch test. If the patient
says they are getting a big stomach, I ask if they can pinch it. If you can
pinch it, it's probably not lipodystrophy, but just fat. Lipodystrophy is
visceral fat -- it is deep, and hard, and different from the subcutaneous
fat you get because you are eating too much, or not getting enough exercise,
or getting older. This is one way to help people feel more comfortable.
Grossman: I see both fat accumulation and fat wasting. Fat accumulation is
sometimes hard to diagnose. I use what I call the pinch test. If the patient
says they are getting a big stomach, I ask if they can pinch it. If you can
pinch it, it's probably not lipodystrophy, but just fat. Lipodystrophy is
visceral fat -- it is deep, and hard, and different from the subcutaneous
fat you get because you are eating too much, or not getting enough exercise,
or getting older. This is one way to help people feel more comfortable.
But I also see many patients with fat accumulation that is lipodystophy. Often the prominent belly, if it is muscular, is not as big a problem for them as facial wasting is for many people. In the gay community some people have eroticized the lipodystrophy look -- facial wasting, prominent cheekbone, veins showing everywhere. But a collapsed-in face is usually much harder for people to deal with than a big abdomen. And doctors who do not ask patients to take their clothes off in the office may not see it. I know many doctors who don't. While there probably is some HIV-related lipodystrophy, and some related to the nucleoside analogs in general, clearly we are seeing something different now than in the past. You sit in a room with 400 patients, and look around, and think, "This is unbelievable." If you live in New York, or San Francisco, or West Hollywood, or probably Philadelphia, where many people walk on the street, you see it. If you live in places where people travel in cars, you don't see how pervasive it is. ATN: What are you using for it? What regimen changes, or treatments? Grossman: Clearly d4T and the protease inhibitors seem to be the bad drugs. Which one causes more, whether it happens mainly with one or the other or is worst with both, we are not sure. My response has been to avoid both, until researchers figure out what is going on. We start almost nobody on protease inhibitors -- unless they come in with a virus with the K-103 mutation, due to transmitted resistance to non-nucleoside drugs. Otherwise I don't touch the protease inhibitors, and I stay away from d4T. Why take that risk for patients when we have other choices? ATN: On resistance testing, what is new that people should be thinking about? Grossman: Sax at Harvard showed that in places where resistant virus is common, as in most of the major cities in the U.S., resistance testing before beginning treatment becomes cost effective [P.E. Sax, #MoPeB3129]. In cost for good outcome, it is comparable to other medical treatments that are well accepted.
We started doing resistance testing before treating people who were chronically infected -- which goes against conventional wisdom. But I think there are enough mutations in the community from AZT, and some of the non-nucleoside and protease inhibitor mutations, that will hang around years after somebody is infected with a resistant virus. This makes it worthwhile to test for resistance, if they were infected in an area with a fairly high prevalence of resistant virus (around 7%). I test them before starting therapy to see if we come up with any antiretrovirals we should avoid. After patients are on therapy, we test everybody who fails. I do both genotyping and phenotyping, because in many patients the picture is complex. You get enough discordance between the genotype and phenotype that having both kinds of resistance testing can be very useful. ATN: Do you use the new ViroLogic service that combines both? Grossman: Yes, the Phenosense GT. Virco has a good test too, but I like the amount of data ViroLogic has on their report. And now they are doing their experimental replicative capacity test as well, looking at the "fitness" of the virus. ATN: What do you think of the fitness test? Grossman: I am going to try it and see. At this point [August 2002] we are just looking to see if it is useful or not. There need to be prospective studies. ATN: What else should patients know about using antiretrovirals? Grossman: It is very important to come back to the doctor for testing when starting certain drugs. With the nevirapine regimens, for example, the company recommends clearly that people get their liver functions tested every two weeks in the first 6 weeks on therapy. We must be careful and catch serious toxicities early. Many patients who start antiretrovirals are used to seeing the doctor every three months. When you start these meds, you need to follow up with the doctor much more frequently at first. Unless the practitioner specifically makes the appointments, patients may not show up again for three months. And in addition to safety, we really want to see viral responses at 4 weeks and 8 weeks and 12 weeks [after starting antiretrovirals]. Especially when someone starts taking antiretroviral medication, I think they should be seen two weeks after they start, to make sure they are doing it right, if nothing else. If they are taking the drugs improperly for months, the chance of developing resistance is high. ATN: Is there anything else you would like to add on antiretroviral therapy? Grossman: The tenofovir data is excellent. The 48 week data from Gilead's trial where it was added to failing therapy had remarkable results [A.L. Pozniak and others, #WeOrB1266]. People maintained their decrease in viral load after 48 weeks, even though their initial baseline therapy had failed to maintain undetectable virus. And Gilead finally released their data in treatment-naive patients, which also shows good efficacy. I am very willing to use tenofovir for first-line therapy. I think it makes so much sense. T-20 On T-20, people need to understand that it takes 30 minutes to mix this drug, which is injected twice a day. You have to put a little bit of fluid in and shake it very carefully, a little bit more and shake very carefully again; you cannot get any bubbles, you cannot bruise the drug because otherwise you denature the protein. It is very complicated to mix, and almost everybody gets injection-site reactions. In studies they found only a 3% drop-out rate due to injection-site reactions; but these were desperate patients who were highly treatment experienced and failing pretty much their last regimen, and T-20 was their last chance. I am not sure what will happen when T-20 comes into wide use. People need to know that while it is a good drug it is certainly not a wonder drug, and it will be very difficult for those people who take it. The company is well aware of that. But the press has not covered how long it takes to mix it; I have not read that anywhere. People need to know that taking T-20 will have an impact on their lives, and they need to think about how they will handle this. You cannot use a mixer or anything that speeds up the process. New data show that both of the day's doses can be mixed in the morning, with the afternoon dose left in the refrigerator. This, at least, will make it a bit easier for those who take it. Prevention ATN: On superinfection -- people with HIV getting infected again with another strain of HIV -- are changes needed in counseling people? Grossman: There were two cases at Barcelona -- the one from Bruce Walker that got all the publicity, and another one from Geneva that was even more impressive because of the testing they did. Clearly both of these people became superinfected with another virus, even though they were doing well with their STI (strategic treatment interruption) regimens, being patients who had been picked up during seroconversion. They suddenly had a burst of virus, and it turned out to be a different virus than they had before. When scientists looked back at their stored samples, the second virus was not there. In the Geneva case the man had been to Brazil and had unprotected sex, and three or four weeks later he had the burst of virus -- and that virus was one typically found in Brazil, not one typical for Geneva. [S. Jost and others, #ThOrA1381] I think it is very worrisome. We were trying to come up with some more personalized sex guidelines for people in the early 90s -- rules that would help people have more protected sex, but that were also more reasonable and individual. So we said, if both of you were positive, maybe it did not matter as much that you use a condom. But I think people got carried away. In New York, San Francisco, Paris, and Los Angeles, many people are not using condoms anymore at all! Many bathhouses are not giving them out; they are not around. We are seeing a rise in new HIV infections across the board in men, especially young men, and we are seeing a rise in other STDs. It is amazing to me how little younger men know about STDs these days. We have been so focused on HIV that we have forgotten to teach about good old syphilis, gonorrhea, warts, etc. And clearly there is a risk of HIV superinfection even if you are both HIV-positive. It may not be a huge, high risk, but the risk is that you could get a second HIV virus and do worse medically. The implications for vaccines are tremendous, because it means that even a well-controlled person who has good CTL immune responses and good responses to the initial virus can be infected with another HIV. ATN: What about oral sex? Grossman: The HOT (HIV Oral Transmission) study, out of San Francisco, is following over 200 men who have sex with men and are believed to have no risk except receptive oral sex [K. Page-Shafer and others, #TuPeC4872]. So far they have found no recent HIV cases. Clearly there have been reported cases. But this is a large study and seems to show that though it does happen, the rate is low. Over the years, Europeans and Australians have focused their prevention messages totally on unprotected anal intercourse, and their HIV incidence rates have dropped tremendously. We gave the mixed messages that we don't know about oral sex, we are not sure, and we've totally confused everyone. As a result, our incidence is high. I think people get frustrated and say, "Nothing is going to protect me, I might as well just do what I'm going to do." Focusing on unprotected anal intercourse is where I think we should be. An epidemiology study in San Francisco found continuing increases in unprotected anal intercourse, including with multiple partners [S. Gibson and others, #MoPeC3430]. There was a big increase in rectal gonorrhea, and a huge increase in early syphilis -- 17 cases in 1997, 10 in 1998, 29 in 1999, 47 in 2000, and 140 in 2001 [figures from the abstract]. HIV incidence also increased greatly, although it may have peaked in 1999. ATN: Overall, what do you think will be most important from the Barcelona conference for treatment of patients with HIV? The main messages from Barcelona were:
AIDS Treatment News Published twice monthly Subscription and Editorial Office: 1233 Locust St., 5th floor Philadelphia, PA 19107 800/TREAT-1-2 toll-free email: aidsnews@critpath.org useful links: http://www.aidsnews.org/ Editor and Publisher: John S. James Associate Editor: Tadd T. Tobias Statement of Purpose: AIDS Treatment News reports on experimental and standard treatments, especially those available now. We interview physicians, scientists, other health professionals, and persons with AIDS or HIV; we also collect information from meetings and conferences, medical journals, and computer databases. Long-term survivors have usually tried many different treatments, and found combinations that work for them. AIDS Treatment News does not recommend particular therapies, but seeks to increase the options available. AIDS Treatment News is published 24 times per year, on the first and third Friday of every month, and print copies are sent by first class mail. Email is available (see below). Back issues are available at http://www.aidsnews.org/ To subscribe, you can call 800-TREAT-1-2 or 415-255-0588: |
 Dr. Howard A. Grossman discusses the recent AIDS conference in Barcelona
Dr. Howard A. Grossman discusses the recent AIDS conference in Barcelona