

Lipoatrophy and Antiretroviral
Drug Changes
|
By John S. James
AIDS Treatment News
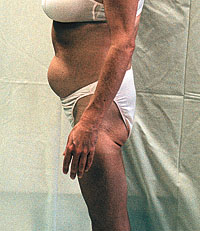 Lipoatrophy is abnormal fat loss, often seen especially in
the face, arms, and legs. Sometimes fat inside the abdomen (a
different kind of fat) will increase at the same time the fat
underneath the skin is disappearing.
Lipoatrophy is abnormal fat loss, often seen especially in
the face, arms, and legs. Sometimes fat inside the abdomen (a
different kind of fat) will increase at the same time the fat
underneath the skin is disappearing.
It is believed that lipoatrophy is caused primarily by antiretrovirals --
although there are different views on whether some protease
inhibitors, some nucleoside analogs, or the combination of
both is most responsible.
Doctors have noted that usually the
lost fat is not quickly regained even if the antiretrovirals
are stopped. Many patients are distressed especially by the
loss of facial fat.
The Retroviruses conference included some reports on partial
success in managing lipoatrophy with certain drug changes.
(See note below on additional information available online.)
[Abstract 32] Patients with moderate or severe lipoatrophy
who were taking either d4T (Zerit(R)) or AZT (Retrovir(R))
were randomly assigned to either continue their treatment, or
substitute abacavir (Ziagen(R)) for the d4T or AZT. This
research team, including leading lipodystrophy researchers
Carr and Cooper in Sydney, Australia, randomized 111 adult
patients. Careful high-tech measurements of limb fat (using
either DEXA or CT) found an improvement in lipoatrophy in the
group that switched to abacavir, but not the group that
continued on their d4T or AZT, in the six months of this
trial. The lost fat returned slowly, however -- patients did
not change their average rating of their own lipoatrophy in
the six months of this trial. And researchers estimated that
at the rate of change which was measured, it would take
several years for the lipoatrophy to disappear.(1)
The patients in this study started with a high CD4 count
(average 577), and a viral load that was stable at under 400
copies with the regimen they were on. Eighty four percent of
them had been taking d4T, 16% had been taking AZT.
The switch to abacavir also resulted in a decline in lactate
(a good sign), and a modest decline in viral load of 0.25
logs.
Other Lipoatrophy Studies
at the Retroviruses Conference
Two Glaxo-supported studies also reported some measurable
improvement in lipoatrophy -- with switches that were
entirely to Glaxo drugs:
[Abstract 701-T] A team at several U.S. universities found
improvements in lipoatrophy when d4T was replaced by either
AZT or abacavir. Of 118 volunteers enrolled, 86 switched to
abacavir, the others to AZT (as Combivir). Lipoatrophy
improvement was found at 24 weeks. The study will run for a
total of 48 weeks.(2)
Note that this study and the one above are similar in one
respect, that both switched most of the volunteers from d4T
to abacavir (except of course for the control group in the
Australian study, which did not switch at all).
[Abstract 700-T] A team in Perth, Australia reported
considerable success with lipoatrophy at 48 weeks, in a
controlled trial in which volunteers were randomly assigned
to either switch from d4T and/or a protease inhibitor to AZT
+ 3TC + abacavir, or not to switch their therapy. Those who
switched did better than those who did not.(3)
[Abstract 684a-T] Another study found a seemingly very
different result. A group of 337 patients who did NOT have
any signs of lipoatrophy were studied 21 months later; 13.1%
of them (44/337) had developed moderate or severe
lipoatrophy. The risk factors were white race, and severity
of disease. When the statistics were adjusted for HIV disease
severity, "there appeared to be little, if any, effect of any
antiretroviral agent or class of agents on the development of
lipoatrophy."(4)
We do not know why this study did not find an association
between development of lipoatrophy and particular
antiretrovirals, while the three studies above had found that
switching antiretrovirals could to some degree reverse
lipoatrophy which had developed on the original treatment
regimen (which would imply that some regimens are more
responsible than others for the development of this
condition).
[Abstract LB13] Rosiglitazone, a diabetes drug which
increases subcutaneous fat in patients with type 2 diabetes,
did not significantly increase subcutaneous fat in a 24-week
trial with 15 HIV patients with lipoatrophy who were on the
drug, compared to 15 controls.(5)
[Abstract 704-T] A cosmetic treatment for facial
lipoatrophy -- injections with polylactic acid (NewFill) --
was reported for 16 patients, 14 men and 2 women, by
physicians in Paris. The doctors reported that the treatment
was "safe, convenient, and effective" in those patients.(6)
Comment: Caution on Switching Drugs
There are many factors to consider in switching
antiretroviral regimens. This decision should be made with
the help of an experienced HIV physician -- who might want to
change other drugs in the regimen to improve antiviral
activity or mitigate other side effects, or to make the
regimen easier to use. Abacavir may not be the best drug for
the particular patient (it just happened to be studied more
and reported at this conference). And if abacavir is used,
the patient and physician must be alert to the
hypersensitivity reaction which occurs in about 5% of
patients; if this happens, the abacavir must be stopped and
NEVER started again.
|
But lipoatrophy is not just a cosmetic problem; it is a sign
that the drug regimen may be causing serious side effects. If
the regimen is not changed, the problem is likely to get
worse. One possible strategy is to act once it is clear that
lipoatrophy is starting, and look for an antiretroviral
regimen that has been shown to stop or slowly reverse this
condition after it has started, in many patients.
For background and cautions on changing therapy, see the
following articles on the lipoatrophy information presented
at this Retroviruses conference:
|
Related Stories from the GayToday Archive:
AIDS Research Today: 20 Views
The 6th Conference on Retroviruses
A Call for More Cautious Antiretroviral Treatment
Related Sites:
AIDS Treatment News
GayToday does not endorse related sites.
|
| |





